Background: The treatment landscape for chronic lymphocytic leukemia (CLL) is evolving at a rapid pace, with development of several targeted therapies. The real-world applicability of clinical trial effectiveness is markedly influenced by treatment choices, the specific characteristics of patients' diseases, their overall health, and individual preferences. However, there is limited evidence to understand the impact of multiple treatment options on real-world effectiveness in CLL in routine clinical practice. This study aimed to characterize recent real-world treatment sequencing patterns and associated time to next treatment among patients with CLL in the United States.
Methods: This retrospective observational study included patients diagnosed with CLL (≥18 years at diagnosis) from January 1, 2018, through January 1, 2023, who received ≥1 treatment after initial diagnosis. Patients were sourced from the ConcertAI RWD360 dataset, a real-world de-identified dataset >6 million patients from across the United States derived from various electronic health record systems and integrated with a large administrative open claims dataset. Patients were followed through the end of the record or death, whichever occurred first. Patients were classified into the following treatment categories: chemotherapy (including bendamustine-based and other chemotherapy), anti-CD20-based, Bruton tyrosine kinase inhibitor (BTKi)-based (ibrutinib, acalabrutinib, or zanubrutinib), and venetoclax-based therapy. Demographic and clinical characteristics, treatment sequencing pattern, and time to next treatment were examined across lines of therapy for each treatment category. Kaplan-Meier analysis was used to estimate median time to next treatment.
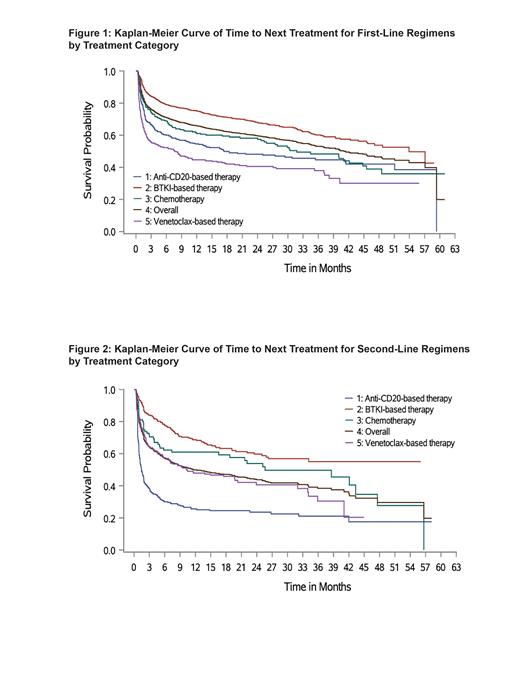
Results: The study included 3,431 patients with CLL (median age: 69 years; male: 63.8%). There was a significant variation in average age among the 4 treatment groups ( P<0.0001). Compared with the overall population, patients who received anti-CD20-based therapy were slightly older (median age: 71 years), while patients who received venetoclax-based therapy were relatively younger (median age: 67 years). Patients were primarily treated in community rather than academic settings (76.4% vs 21.3%). First-line patients were most commonly treated with BTKi (60.1%), venetoclax (16.0%), and anti-CD20 (15.4%) regimens. Among patients who initiated BTKi therapies, zanubrutinib use (2.6%) was limited; this may be due to the recent approval date (January 2023). Among CLL patients, 35.0% received second-line treatment and BTKi stayed dominant (35.7%), followed by anti-CD20 (29.6%) and venetoclax (25.8%) therapies. From first- to second-line therapy, the prevailing sequencing pattern was from either one BTKi to another BTKi (23.5%), venetoclax to anti-CD20 (13.2%), or BTKi to an anti-CD20 (9.7%). From second- to third-line therapy, patients most frequently switched from anti-CD20 to venetoclax (24.6%), anti-CD20 to BTKi (13.3%), or one BTKi to another BTKi (12.6%). From third- to fourth-line therapy, the predominant sequencing pattern was from venetoclax to anti-CD20 (21.3%), anti-CD20 back to venetoclax (20.6%), or one BTKi to another BTKi (11.6%). Median time to next treatment in first line was significantly longest for patients receiving BTKi (53.9 months) and shortest for patients treated with venetoclax (7.7 months) ( P<0.0001) (Figure 1). Median time to next treatment in second line was not reached among patients using BTKi, while it was significantly shorter for those on venetoclax and anti-CD20 (10.4 months and 1.5 months, respectively; P<0.0001) (Figure 2).
Conclusions: This studydemonstrated unique treatment sequencing patterns among patients with CLL in the real-world setting.As the most commonly used regimen, BTKi therapy was shown to have significantly improved time to next treatment compared with other treatment regimens in first and second lines of therapies. Future studies with extended follow-up are needed to allow for assessment of newer treatments and enable evaluation of long-term clinical outcomes.
Disclosures
Chanan-Khan:Ascentage: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Mayo Clinic: Current Employment; BeiGene: Consultancy, Honoraria; Cellectar: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Starton: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Alpha2: Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Challagulla:Abbvie: Ended employment in the past 24 months; BeiGene: Current Employment, Current equity holder in publicly-traded company. Miller:Merck, Bristol-Myers Squibb, Incyte, Gilead Sciences, AbbVie, Astellas Pharma, Daiichi Sanko/UCB Japan, AstraZeneca, Janssen Oncology, Sandoz: Consultancy. Wang:ConcertAI, AbbVie, Merck, Merck KGaA, BMS, Astellas, Kyowa Kirin, Sandoz, Incyte, Beigene, Teva: Consultancy, Current Employment. Smith:Amgen: Research Funding; Bristol Myers Squibb: Research Funding; Gilead: Research Funding; Incyte: Research Funding; Merck: Research Funding; Novartis: Research Funding; AstraZeneca: Research Funding; AbbVie: Research Funding; Astellas: Research Funding; Daiichi Sankyo: Research Funding; Janssen: Research Funding; Kyowa Kirin: Research Funding; Boehringer Ingelheim: Research Funding; GlaxoSmithKline: Research Funding; Sandoz: Research Funding; Teva: Research Funding. Yang:BeiGene: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding.